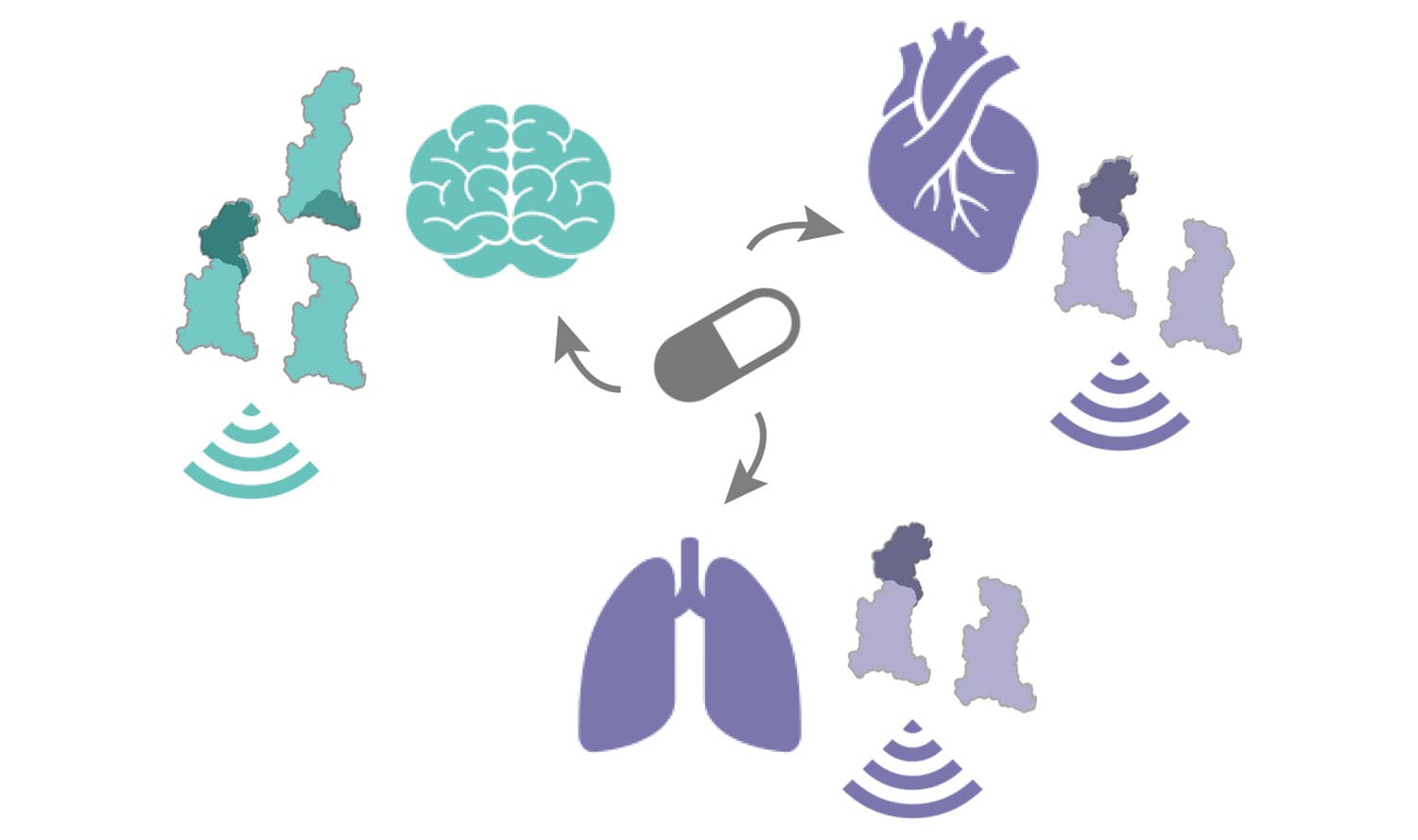
Important drug target receptors exist in multiple structurally and functionally distinct versions distributed in a tissue-specific manner around our bodies
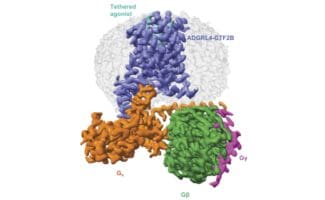
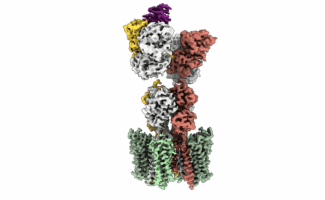
One family of proteins, called G protein-coupled receptors (GPCRs), is the target of approximately 34% of approved drugs. This reflects the great variety of signals that these receptors detect at the cell surface, such as hormones, neurotransmitters, ions, and light, and their key role as modulators of cell function in response to these signals. While at the LMB, Madan Babu’s group, in the Structural Studies Division, investigated how different versions of the same GPCR are distributed across human tissues by integrating multiple sources of big data. This gave the team a detailed understanding, on a tissue-by-tissue basis, of natural receptor activation and drug effects.
An important challenge in drug design against GPCRs is that relatively small changes in the 3D structure of receptors can change the way in which these receptors bind to drugs and activate a cell response. These changes in receptor 3D structure not only arise from genetic differences between individuals in the population but can also occur in a single person due to the natural formation of alternative versions of the same receptor by processes such as alternative splicing.
Maria Marti-Solano, a Marie Skłodowska-Curie postdoctoral researcher in Madan’s group, wanted to investigate how these naturally occurring receptor versions, also known as receptor isoforms, are distributed across human tissues. Knowing the differences in the structure and tissue location of the different receptor isoforms can help understand whether activating the same receptor in different parts of our body can result in responses that are tissue‑specific.
In this study, Maria and her collaborators integrated and analysed genomics, transcriptomics, proteomics, structural, and pharmacological data for more than 300 receptors in 30 different tissues from more than 750 donors. This investigation created a vast amount of data that was used to generate a new resource in the GPCR database maintained at the University of Copenhagen (David Gloriam). This resource will allow experts interested in particular GPCRs to determine if their receptor of interest can exist in one or multiple isoforms and get detailed information of receptor isoform structural and functional data on a tissue-by-tissue basis.
This work uncovered a new layer of complexity in receptor function that can change the way scientists approach the study of GPCR signalling. This is due to the finding that diverse isoforms can be found in different combinations in different tissues, meaning that the observed outcome of a signal reaching a tissue can be the result of the combination of responses from different GPCR isoforms. This means that, in order to design experiments to understand GPCR function in human tissues, scientists may need to incorporate to their studies not one, but a collection of receptor isoforms found in the tissue they want to analyse. To experimentally validate some of these findings, collaborators from the University of Michigan (Manoj Puthenveedu), University of Cambridge (Graham Ladds) and the University of Glasgow (Graeme Milligan and Andrew Tobin) characterized the signaling response of the isoforms and their combinations for some of the receptors analysed in this study.
This study also offers new information on which isoforms of a GPCR are found in different parts of the body. This, combined with the different 3D structure of these isoforms, could make it possible to design drugs that bind specific isoforms found only in the tissue that we need to treat, and that avoid the unwanted side effects that arise from activating other isoforms of the same GPCR elsewhere in the body.
The work was funded by UKRI MRC, Wolfson College, FEBS, Marie Skłodowska-Curie actions (see below), Swiss National Science Foundation, NIH, NIDA Core Center of Excellence in Omics, Systems Genetics, and the Addictome, NSF, UKRI BBSRC, AstraZeneca, Lundbeck Foundation, Novo Nordisk Foundation, Lister Institute, ERC, and ALSAC.

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 832620.
Further References
- Combinatorial GPCR isoform expression impacts signalling and drug responses. Marti-Solano, M., Crilly, SE., Malinverni, D., Munk, C., Harris, M., Pearce, A., Quon, T., Mackenzie, AE., Wang, X., Peng, J., Tobin, AB., Ladds, G., Milligan, G., Gloriam, DE., Puthenveedu, MA., Babu, MM. Nature.
- Madan’s group page
- Manoj Puthenveedu’s group page
- David Gloriam’s group page
- Graeme Milligan’s group page
- Graham Ladds’ group page
- Andrew Tobin’s group page
- Junmin Peng’s group page
- GPCR database
Nature News & Views: Isoforms of GPCR proteins combine for diverse signalling