I became a Staff Scientist at the LMB in 1968, after obtaining a PhD from the University of Cambridge. In 1980, I left to become Professor of Cell Biology at Stanford University, California, and returned in 1988. In 1992, I became Head of the Neurobiology Division, followed by a position as Joint Head from 2003 until 2008.
I have been interested in developing electron microscopical methods and using them to analyse the structures of proteins in membranes. In 1975, together with Richard Henderson, I determined the first structure of an integral membrane protein: bacteriorhodopsin.
Since then, a major focus of my research has been the nicotinic acetylcholine receptor – the transmitter-gated ion channel at the nerve-muscle synapse. In 2005, I obtained an atomic model of this channel in its native postsynaptic membrane. Later, time-resolved spray-freezing experiments elucidated how the channel opens transiently upon brief exposure to acetylcholine. This event takes place by an asymmetric conformational change involving small movements of α-helices encircling the central membrane-spanning pore.
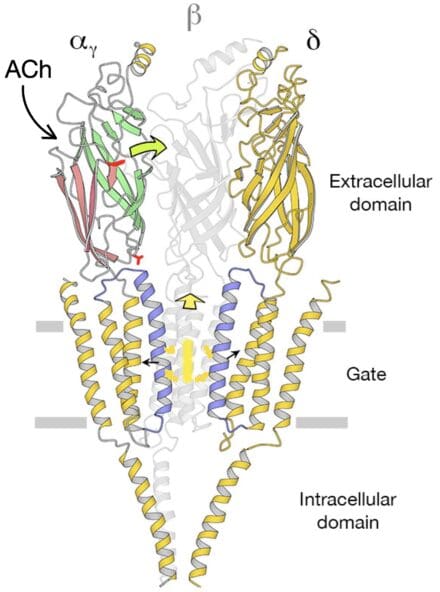
Recent experiments suggest that acetylcholine receptors exploit a special lipid environment at the synaptic junction to fine-tune the conformational change and optimise the postsynaptic response. I am currently using cryo-EM to explore the molecular nature of this protein-synaptic lipid interplay.