I came to the LMB as a postdoc with Hugh Huxley in 1973, after obtaining my PhD and working for two years at the University of New South Wales on carbon fibres. After three years with Hugh, working primarily on the structure of tropomyosin and its interactions in muscle thin filaments, I returned to Australia, where I worked at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) on image processing and biological problems. I returned to the LMB in 1981 as a Group Leader, initially focussing on muscle and cell motility before concentrating on the molecular mechanism of nucleocytoplasmic transport. I was elected a member of EMBO in 2006.
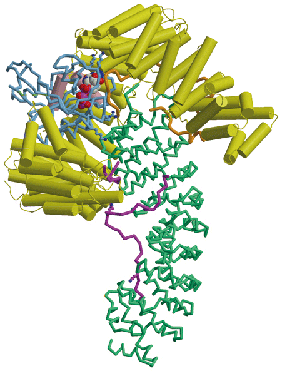
Proteins and nucleic acids are transported between the cytoplasm and nucleus through nuclear pores by carrier molecules that can overcome the barrier function generated by unfolded regions of proteins (FG-nucleoporins) that fill the nuclear pore transport channel. The binding or release of cargoes is often orchestrated by the Ran GTPase. My research aims to establish the structure of the proteins involved and how they interact to generate transport. The structures of a range of karyopherin-family transport factors, that transport primarily proteins and small RNAs, together with their complexes with nuclear pore proteins, cargoes and Ran, have been established using X-ray crystallography. These structures have been used to engineer mutants that have identified the importance of karyopherin flexibility that enables RanGTP to mediate cargo binding and release.
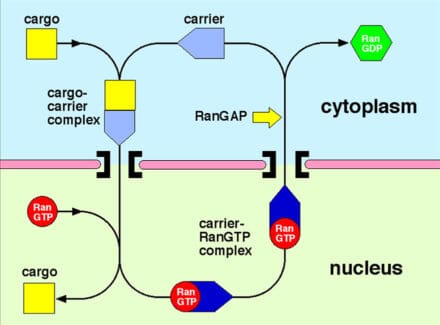
My current research concentrates on the nuclear export of mRNA that uses the Mex67-Mtr2/NXF1-NXT1 family and DEAD-box ATPases instead of Ran. I aim to understand how nuclear trafficking components, the TREX and TREX-2 complexes, the polyA-binding protein, Nab2p and the Mex67-Mtr2 transport factor, orchestrate passage of mRNAs through the nuclear gene expression machinery to the cytoplasm, especially in the context of their role as scaffolds for the formation of the large macromolecular complexes involved in splicing, mRNA processing and nuclear export.