David Barford
Mechanism and regulation of chromosome segregation in mitosis

Our research aims to understand the mechanisms and regulation of chromosome segregation in mitosis. Accurate chromosome segregation ensures the correct inheritance of genetic material and organismal survival. Errors in this process cause aneuploidy, leading to cancer and developmental defects. At metaphase, chromosomes align on the metaphase plate so that each sister chromatid is attached to microtubules by kinetochores, large protein complexes that assemble at the centromere. Once all chromosomes attach to the spindle and tension is exerted at the kinetochore-microtubule attachment site, anaphase is triggered. This results in the segregation of sister chromatids, a process powered by microtubule depolymerisation. Anaphase onset is triggered by the anaphase-promoting complex APC/C, a large multi-subunit complex that functions as an E3 ubiquitin ligase to regulate different stages of the cell cycle.
Our current focus is to understand the process of chromosome segregation mediated by the kinetochore. We are undertaking a programme to determine the kinetochore’s molecular and cellular structure and mechanism. We use a variety of approaches, including complete reconstitution of kinetochores and their attachment to chromosomes and microtubules, for analysis by cryo-EM and biophysical methods such as optical tweezers.
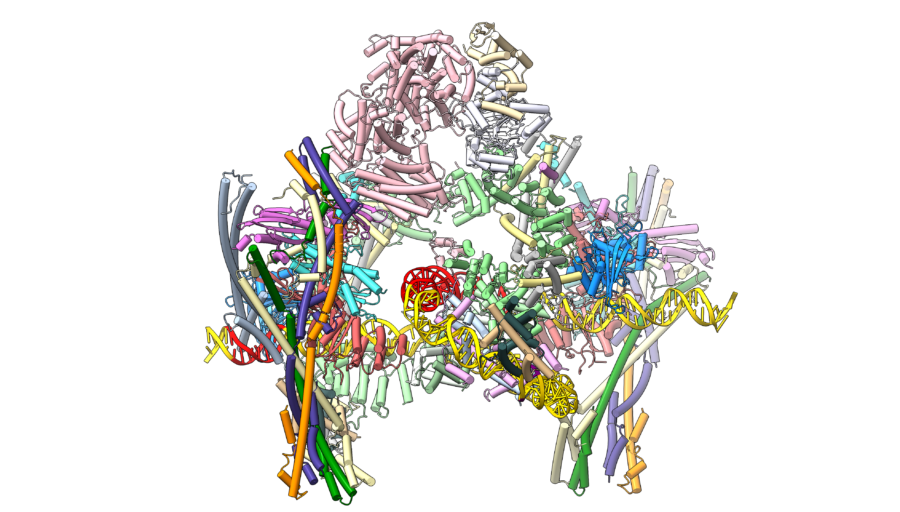
We also investigate the structure of the kinetochore in situ using electron cryotomography (cryo-ET). We aim to determine cryo-EM structures of complete reconstituted kinetochore complexes that will aid their visualisation at centromeres in cells. We also seek to understand the spindle assembly checkpoint and error correction checkpoint from a structural, biochemical and biophysical mechanistic perspective.