G protein-coupled receptors (GPCRs) are integral membrane proteins found throughout the human body and are the cornerstone of intercellular signalling. They bind molecules such as hormones, undergo a conformational change and then activate either G proteins or β-arrestins, which then activate various intracellular signalling cascades that alter the biochemistry of the cell. GPCRs are the targets of 34% of marketed small molecule drugs, which include beta blockers and treatments for asthma, high blood pressure, migraines and chronic pain.
Our group has used X-ray crystallography and cryo-EM to determine over 40 structures of GPCRs bound to different ligands, in different conformational states or bound to different transducers (G protein or β-arrestin). The structures have elucidated the molecular basis for fundamental pharmacological properties of GPCRs, such as ligand specificity and efficacy, the specificity of G protein and β-arrestin coupling and the molecular basis for the consequent increase in agonist affinity.
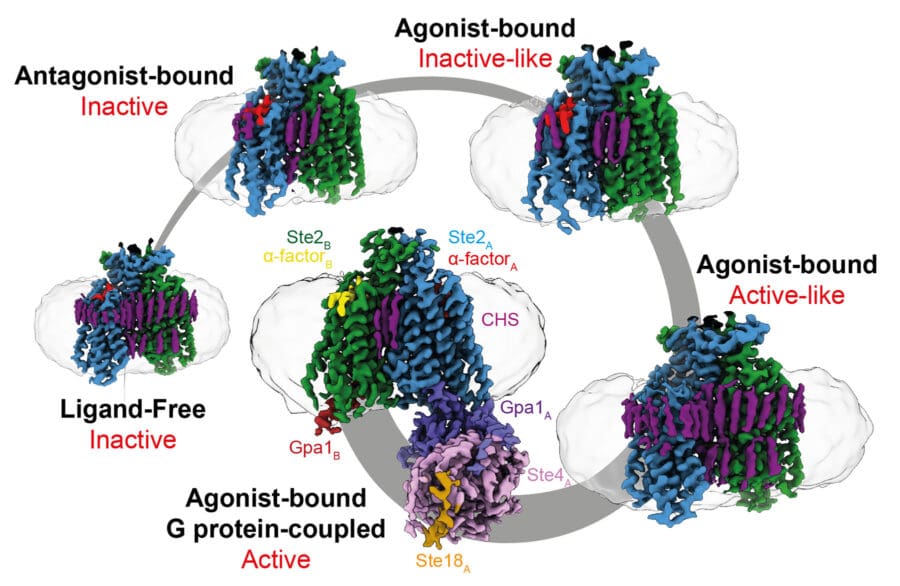
Recently, we have turned our attention to GPCRs in fungi, which are structurally poorly characterised and little studied. We determined the first structure of a fungal GPCR, which revealed many surprises, including the structure of the dimeric interface, its ability to couple to two G proteins simultaneously, differences in transmembrane helix arrangement compared to mammalian GPCRs and how it is activated. Our future studies will focus on receptors in pathogenic fungi, to facilitate drug development to combat intractable systemic fungal infections in humans, animals and plants.