
After studying chemistry at the University of Oxford and then obtaining a PhD in organic synthesis at Imperial College London while part-running the departmental nuclear magnetic resonance (NMR) service, I switched full-time to NMR, working with Kurt Wüthrich at ETH Zürich when his group was first developing methodology for solution-phase protein structure determination. My work there included some of the first 1H-detected, heteronuclear experiments on proteins – forerunners of techniques that today are central to biological NMR structure determination. After a second postdoc, with Ray Freeman in Oxford, I moved to Cambridge and in 1988 joined the LMB to introduce NMR to the Lab.
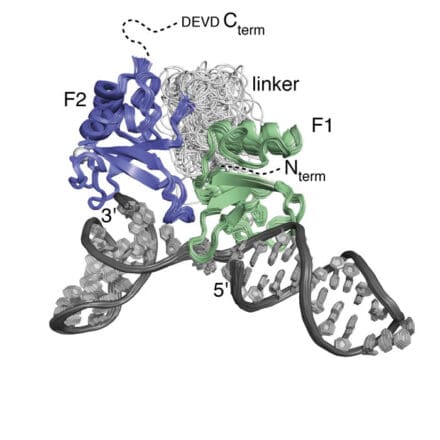
While at the LMB I collaborated widely, both internally and externally, on diverse and challenging structural projects, always seeking to use NMR where it could bring the strongest benefit. Starting in the early 1990s with zinc finger domains from transcription factors (oestrogen receptor and SWI5), work on DNA and RNA recognition by proteins continued to be a theme, including particularly the first NMR structures of protein-RNA complexes with Gabriele Varani and Kiyoshi Nagai. Other projects included work on protein folding, nuclear trafficking, amoeboid motility, the F1-ATPase system, RNA splicing, chromatin targeting, vesicle trafficking, mating type determination in Dictyostelium discoides and intracellular immunity.
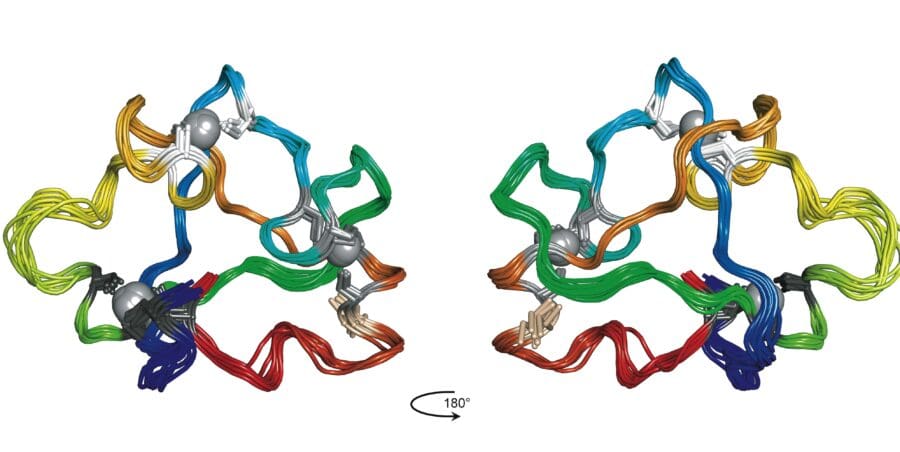
A particular focus recently has been work on the human DNA repair factor PARP-1, the principal sensor of DNA single-strand breaks and an important cancer target. Using NMR with X-ray crystallography and other biophysical techniques, and in collaboration with groups in Montréal, AstraZeneca and Oxford, we showed how PARP-1 recognises single-strand breaks, and how this causes a domain assembly cascade within the protein-DNA complex that triggers activation, starting synthesis of poly(ADP-ribose), or PAR, the signal for further assembly of the repair complex.
I am a Fellow of the Royal Society of Chemistry and chaired its NMR Discussion Group 2001-2003. I have served on several international advisory and editorial boards, and have run two invited review journals: Quarterly Reviews of Biophysics (1997-2002) and Progress in NMR Spectroscopy (2012-present). Together with Mike Williamson, I co-authored the standard text on the nuclear Overhauser effect, the phenomenon that underlies the ability of NMR to solve three-dimensional biomolecular structures.