
I began my research career at the University of Birmingham, studying contractile proteins in developing skeletal muscle. During my postdoc at Brandeis University in Boston, I investigated how muscle contraction is regulated and uncovered a novel myosin-linked calcium regulatory pathway, challenging the belief that troponin was the sole calcium regulator of muscle contraction.
In 1970, I joined the LMB and continued exploring myosin regulation. I identified small subunits associated with the myosin motor domains, later named regulatory light chains. I demonstrated that all myosin II molecules contain these regulatory light chains, which play a crucial role in motor function and filament assembly across both muscle and non-muscle cells.
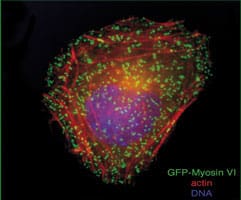
In the early 1990s, together with other groups, we established that myosins form a superfamily consisting of 18 major classes of motor proteins. More recently, I have focussed on class VI myosins, revealing their multifunctional roles in a wide range of cellular processes.
Abnormal protein trafficking pathways in cells play a key role in many human diseases. Understanding how motor proteins function within these pathways is crucial for uncovering disease mechanisms and identifying potential therapeutic targets. I have focussed on characterising the intracellular functions of myosin VI—the only myosin that moves towards the minus end of actin filaments.
We have shown that myosin VI, through interactions with specific adaptor proteins, plays essential roles in cellular processes including actin filament organisation and cell movement, endocytosis and autophagy. Mutations or overexpression of myosin VI have been linked to a number of pathologies including deafness, neurodegeneration and cancer. The goal is to define the intracellular functions of myosin VI and to understand its involvement in these disease processes.