Lalita Ramakrishnan
Mechanisms of tuberculosis pathogenesis

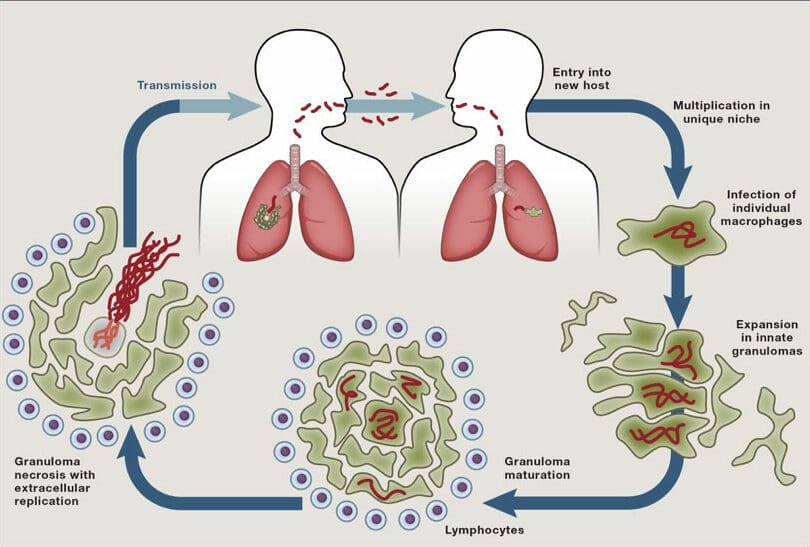
Tuberculosis (TB) remains a major cause of death despite the existence of a live attenuated vaccine (BCG) for a century and effective antibiotics for 60 years. TB’s persistence over millennia in the face of these major medical advances underscores the ability of Mycobacterium tuberculosis to evade and exploit host defences and antibiotics. Pathogenic mycobacteria infect macrophages and survive in these primary immune defence cells by subverting their endocytic trafficking and microbiocidal mechanisms. The bacteria also alter macrophage differentiation, migration and aggregation to induce the formation of granulomas, the enigmatic, complex, organised immune structures that can paradoxically promote bacterial growth. Ultimately, mycobacteria can cause granuloma breakdown by inducing programmed macrophage death, a step that further increases bacterial growth and promotes transmission to new hosts.
We use zebrafish larvae as a surrogate model organism to study TB, exploiting its optical transparency and genetic and pharmacological tractability to monitor infection in real time. Through genetic screens, we identify host susceptibility and resistance factors and bacterial virulence determinants that alter specific infection steps. Our research is shedding light on TB pathogenesis as well as fundamental macrophage biology – organellar cross-talk, migration, adhesion and death. Our findings have been borne out in humans and are informing new treatment strategies. We have also used the zebrafish to unravel mysteries in other granulomatous diseases like leprosy and schistosomiasis.