Kelly Nguyen
Molecular mechanism of telomere maintenance

Eukaryotic chromosome ends are composed of repetitive, G-rich DNA sequences (TTAGGG in humans) known as telomeres. These structures are essential for maintaining genome stability and cell survival. Telomere dysfunction has been linked to ageing and cancer. Our group focusses on understanding how cells address two major challenges: telomere shortening due to incomplete DNA replication (end replication) and preventing chromosome ends from being mistakenly recognised as DNA breaks (end protection). To tackle these questions, we use a combination of biochemistry, structural biology, and functional in-cell studies.
To counteract telomere shortening, a specialised reverse transcriptase called telomerase synthesises telomeric repeats de novo at chromosome ends. Telomerase activity is crucial for the sustained proliferation seen in immortal cells, such as cancer cells, stem cells and germline cells. Conversely, mutations that impair telomerase function can lead to premature ageing. Our group has determined several cryo-EM structures of human telomerase, providing key insights into its architecture, mechanism, and recruitment. Building on these advances, we continue to investigate fundamental questions regarding the evolution, regulation and assembly of telomerase.
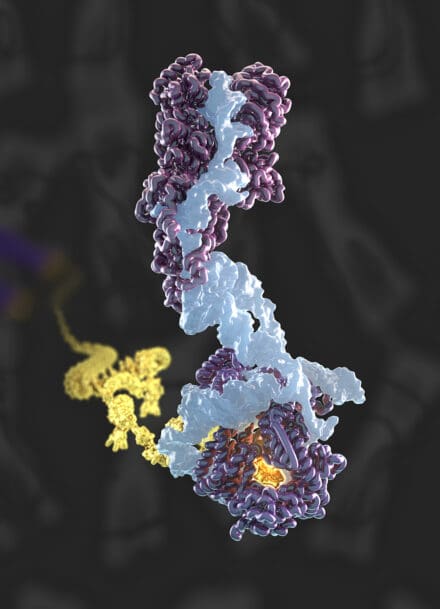
In parallel, chromosome end protection is ensured by telomere-associated protein complexes, which prevent telomeres from being mistaken for DNA damage. However, the structural details of these complexes and their role in organising telomeric chromatin remain largely unknown. Our goal is to elucidate the structures of these telomeric complexes and ultimately whole telomeres, to gain a deeper understanding of the mechanisms underlying end protection.