Andrew Carter
The structure and mechanism of dyneins

The contents of eukaryotic cells are organised and moved around by motor proteins running along the tracks that make up the cytoskeleton (microtubules and actin filaments). The largest and most complicated of these motors is cytoplasmic dynein. It is involved with numerous processes, from positioning organelles to cell division and clearing up misfolded proteins. Defects in dynein are linked to developmental disorders and neurodegeneration. Dynein is also used by viruses such as herpes and rabies to infect the cell. Our goal is to understand how dynein contributes to medically relevant, cell-biological processes.
We initially used X-ray crystallography to solve the structures of dynein’s motor, which explained how it uses ATP to move along microtubules. We have since taken advantage of advances in cryo-EM to understand how dynein, and its cofactor dynactin, bind the adaptors that link them to cargoes. We showed how the formation of dynein-dynactin-adaptor complexes activates long-distance transport and how this process is controlled by the dynein regulator LIS1.
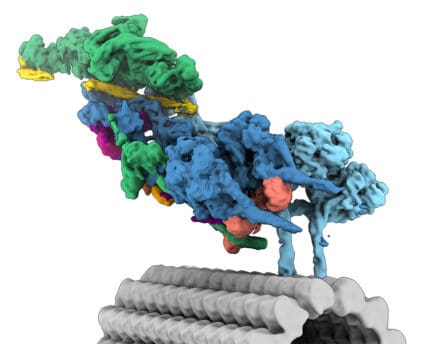
We are interested in the many structural and mechanistic questions that surround dynein transport. How can one dynein complex carry so many different cargoes? What mechanisms control whether dynein or the opposite direction kinesin motors are engaged? How do viruses and other pathogens hijack motors? We will combine biophysical and cell biological methods with the latest techniques in structural biology to answer these.