The overall goal of our research is to understand the molecular mechanisms involved in the initiation of immune responses by dendritic cells (DCs). The critical role of DCs in the immune system depends on their ability to sense diverse pathogen- and damage-associated molecular patterns (PAMPs or DAMPs) and stimulate pathogen- or cancer cell-specific naïve T cells to become cytotoxic effectors.
One of the key questions we focus on is how DCs acquire, process and present antigens from surrounding cells on their MHC class I molecules. This process is referred to as cross-presentation and is critical for initiating immune responses against antigens not expressed in DCs, such as neoantigens present in cancer cells. To gain insights into the mechanisms underlying cross-presentation, we employ a combination of CRISPR-Cas9-based screens, microscopy, proteomics and animal models.
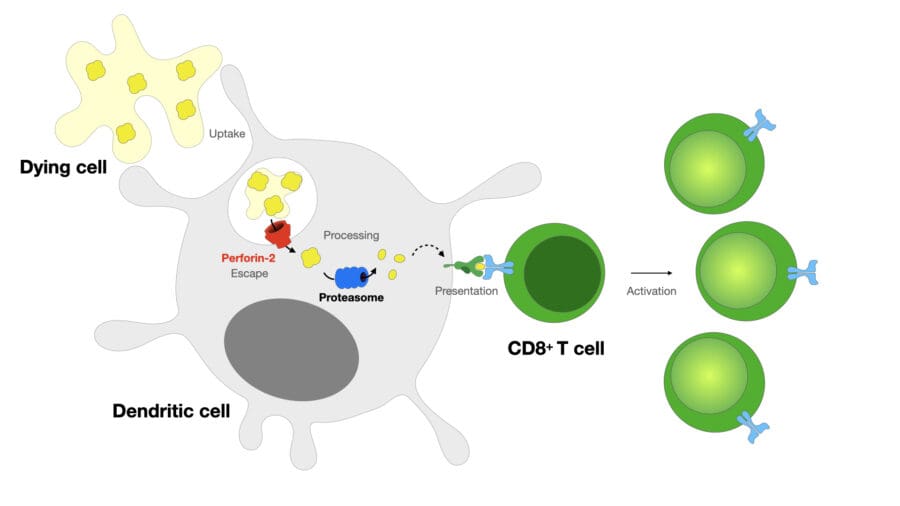
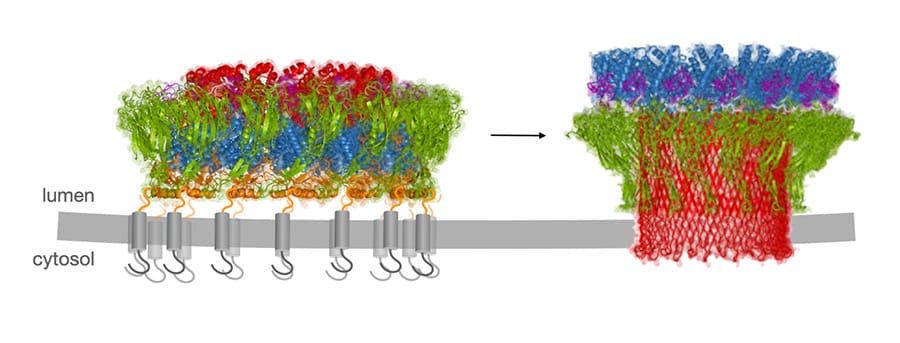
We recently demonstrated that cross-presenting DCs express a pore-forming protein, perforin-2. Perforin-2 is recruited to antigen-containing compartments, facilitating the escape of antigens into the cytosol, where they are processed by proteasomes for presentation on MHC class I. Our current focus is on the mechanistic studies of perforin-2 regulation and on the role of endocytic escape pathways in vivo.