Marta Shahbazi
Cell fate decisions in the early mammalian embryo

Embryo development starts with the zygote, a single cell that gives rise to the myriad of cell types present in the adult organism. The main goal of our group is to understand how different cells in the early embryo decide to take on different specific identities and functions. Such fate decisions do not occur in isolation, but within an embryo that is acquiring specific shapes. We are studying the mechanisms that ensure the right cell types arise at the right place and at the right time. We focus on the critical stage of mammalian development when the embryo is implanting into the mother’s uterus. This stage is important because it is when the basic body plan is formed and when approximately 30% of human pregnancies fail.
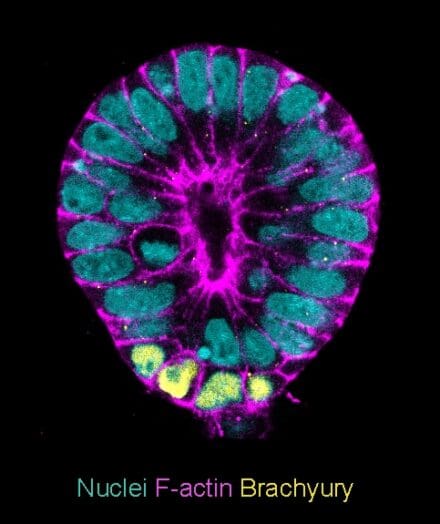
Failure of pregnancies can be due to a range of developmental and cellular errors, some of which occur remarkably frequently. For example, aneuploid cells, which contain an abnormal number of chromosomes, are found in approximately 80% of early human embryos. We seek to understand how embryos can sometimes overcome these mistakes and develop normally. To investigate this problem, we analyse supernumerary human embryos from assisted reproductive techniques that are donated for research under a licence from the Human Fertility and Embryology Authority.
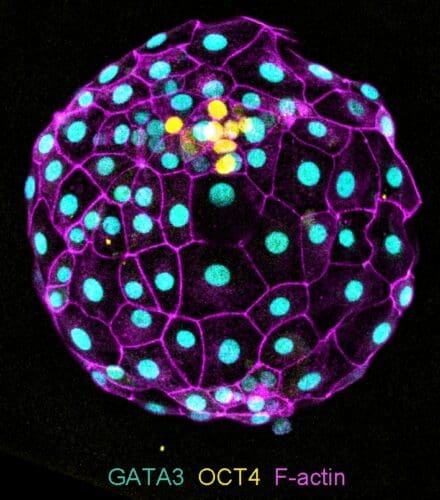
Through our work on both normal and aberrant early human embryogenesis, we aim to understand our developmental origins and how errors during these crucial first days impact human development.