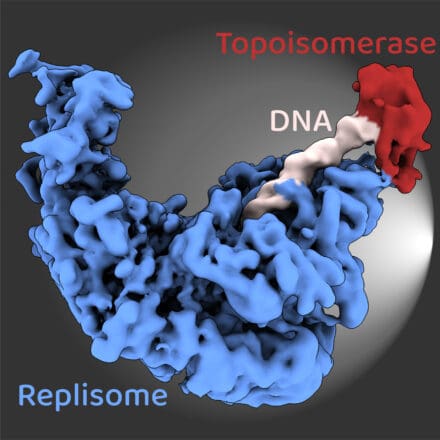
When our cells divide, they must first duplicate their chromosomes, which contain the six billion base pairs of DNA that make up the diploid human genome. Just a few copying errors can cause mutations that drive cancer development. The mechanisms that underpin accurate DNA replication are therefore vital for human health and disease. DNA replication is performed by a large and complex ensemble of molecular machines, collectively termed the replisome. Our group provides fundamental new insights into the mechanisms of the replisome by combining biochemical reconstitution with structural biology and functional assays.
Replisomes unwind the double-stranded DNA template and coordinate the synthesis of two new DNA strands, which are extended in opposite directions and by different DNA polymerase enzymes. To study the mechanics of this process, we recently reconstituted functional human replisomes from purified proteins. Work is now focussing on how DNA unwinding is coupled to DNA synthesis to control rates of replisome progression, and how replisome accessory proteins modulate replication speed.
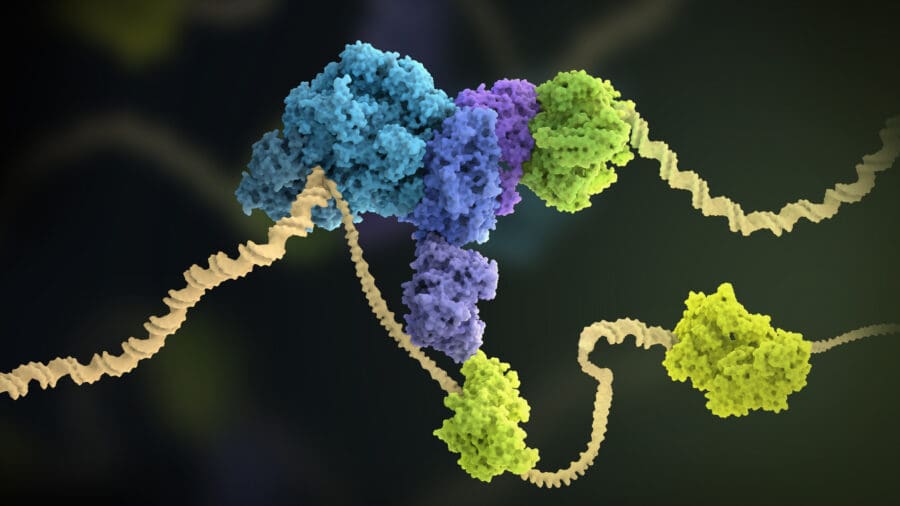
Replisome progression along chromosomes is frequently interrupted by tightly-bound protein complexes, structured DNA and DNA damage. For chromosome replication to be completed without the loss of genetic information, these interruptions must all be overcome. To achieve this, the replisome is assisted by a wide variety of accessory proteins and the DNA repair and recombination machinery. We have a long-standing interest in understanding how these proteins and pathways interface with the replisome to promote continued DNA synthesis and the timely completion of chromosome replication.