Cryo-EM structures of a principal AMPA receptor complex in open and closed states provide the first insight into how two major auxiliary subunits modulate receptor function
Our brains are made up of networks of neurons where each individual nerve cell can make connections with thousands of others. AMPA receptors are a set of membrane proteins that mediate fast signal transmission across these connection points called synapses, with certain receptor complexes predominating in different parts of the brain. Ingo Greger’s group, in the LMB’s Neurobiology Division, has visualised an AMPA receptor with two different auxiliary subunits, a specific combination that is enriched in the hippocampus and involved in learning and memory.
AMPA receptors are found on the post-synaptic membrane of excitatory synapses, where the signal is received and must be propagated. In particular, AMPA receptors are responsible for most of the fast excitatory synaptic transmission in the brain. A key feature of AMPA receptors is that they are complexes that can be assembled from a number of combinations of different subunits and can be modulated by different auxiliary subunits, allowing differential regulation across cell-types and regions of the brain. How different auxiliary subunits co-operate to increase ion flux through the receptor channel has so far been elusive.
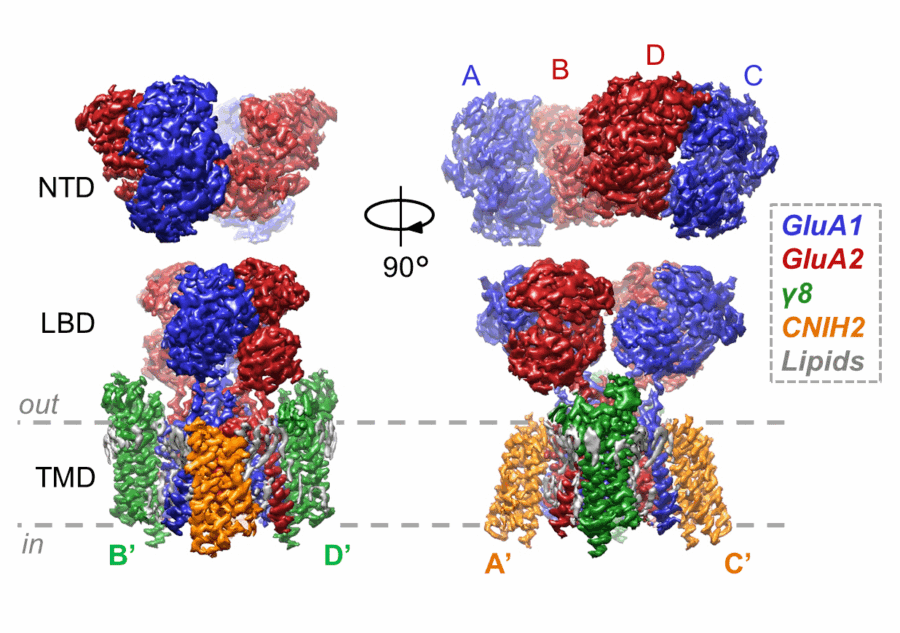
The most common AMPA receptor complex found in the forebrain and hippocampus consists of two copies of the GluA1 subunit and two copies of the GluA2 subunit associated with two copies of the auxiliary subunits TARP-γ 8 and CNIH2. Two years ago, Ingo’s group revealed a structure of this AMPA receptor with TARP-γ 8 in a closed state, providing some insight into this auxiliary subunit’s function. His group have now revealed structures of the same AMPA receptor, with both of these auxiliary subunits, in an active, open state and in a resting, closed state, providing a more complete understanding of how this particular AMPA receptor complex functions.
Danyang Zhang, a postdoctoral researcher in Ingo’s group, determined these structures using cryo-EM. By comparing the open and closed states, the team then produced a model to explain how these auxiliary subunits promote the open-channel state to increase ion flux. The structures also provide unprecedented insight into how the receptor transitions between open and closed states.
As the primary receptor mediating fast excitatory synaptic transmission throughout the forebrain, malfunction of this particular AMPA receptor can lead to various neurological and neuropsychiatric disorders, such as epilepsy and depression. As well as providing insight into function, these high-resolution structures visualise cavities and lipids on the receptor that could aid development of drugs specific to this receptor.
The work was funded by UKRI MRC and BBSRC.
Further references
Gating transitions and modulation of a hetero-octameric AMPA glutamate receptor. Zhang, D., Watson, JF., Matthews, PM., Cais, O., Greger, IH. Nature
Ingo’s group page