
I started my career as a PhD student with Max Perutz in 1965, working on CO2 transport by haemoglobin and the Bohr effect. I prepared modified haemoglobins to test whether particular amino acids, some predicted from the X-ray crystallographic structure Perutz had just solved, were involved in these effects.
In 1976 I changed direction and began working on molecular aspects of yeast mitosis, using both genetic and biochemical methods. I purified tubulin from yeast, made monoclonal antitubulin antibodies and developed immunofluorescence methods for yeast. Immunofluorescent screening of temperature-sensitive mutants identified a mutant, ndc10-1, in a kinetochore protein. The biochemical approach led to highly enriched spindle poles, containing the yeast equivalent of the centrosome, the spindle pole body (SPB). This allowed the identification of numerous components of both the spindle and the SPB, first from monoclonal antibodies and later by mass spectrometry. In addition, using a pulldown, I identified a low-abundance protein, Sfi1, which has a critical role during SPB duplication.
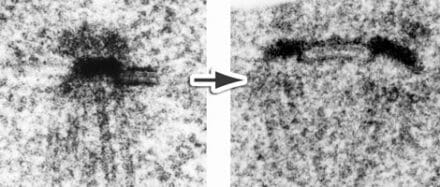
Sfi1 is a filamentous protein that binds SPB components at its N-terminal end and spans entirely a specialised part of the nuclear envelope called the half-bridge, with the C terminus at the distal end. At the start of SPB duplication, the half bridge doubles in length due to an end-to-end dimerisation of Sfi1 at the C terminus. This gives a new Sfi1 N terminus that can assemble the daughter SPB. More recently, in collaboration with David Barford’s group in the Structural Studies Division, I have been looking at the cryo-EM structure of the SPB. In particular, we looked at the g-TuRC, the structure that initiates spindle microtubule assembly, and we found a novel interaction between a coiled-coil protein and the exterior of the γ-TuRC.