Roni Levin Konigsberg
Molecular physiology of phagocytic cells in health and disease

Our bodies are continuously challenged by external and internal microscopic materials that include micro-organisms, ‘bad’ cholesterol, dead cells and protein aggregates. Over 200 billion dying cells must be cleared from our bodies daily. If these materials are not promptly cleared from our system, they can lead to a wide range of diseases that include infections, heart disease, storage disorders and neurodegeneration.
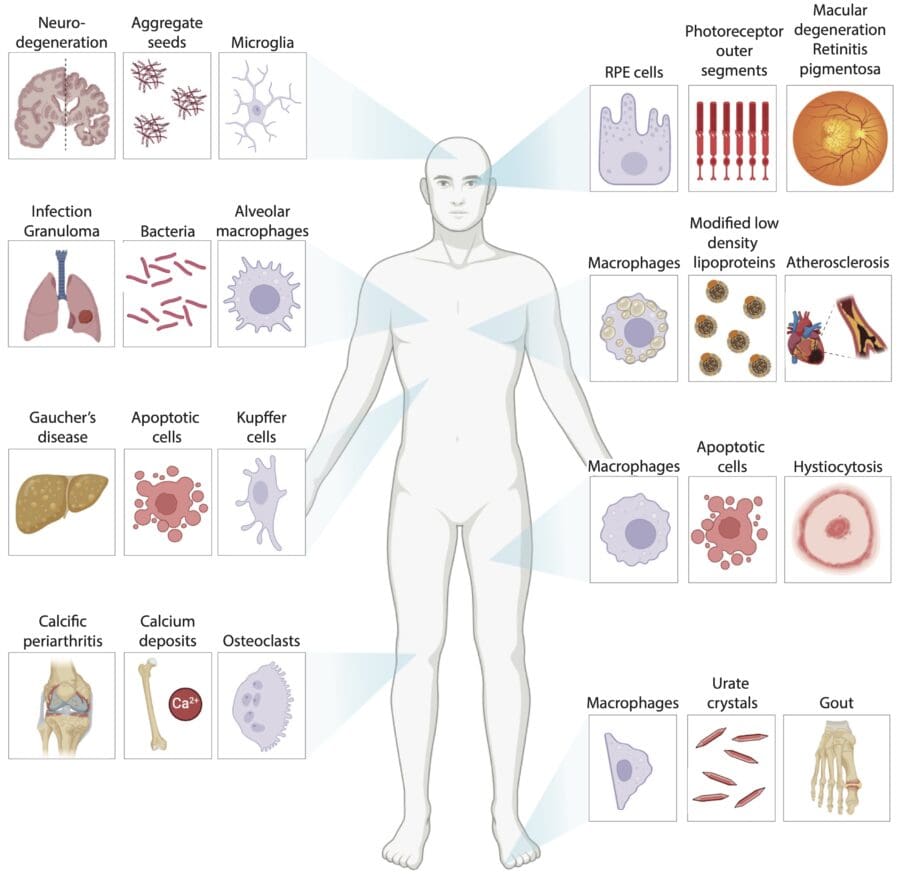
Specialised cells called phagocytes, which include immune cells such as macrophages and microglia, are tasked with the recognition, clearance and degradation of potentially harmful particulate materials from our tissues and circulation. Our group aims to understand the molecular mechanisms that allow phagocytes to ‘eat’ and process very large numbers of widely diverse materials on an ongoing basis while remaining healthy and functional throughout their lifespan, which can range from months to years.
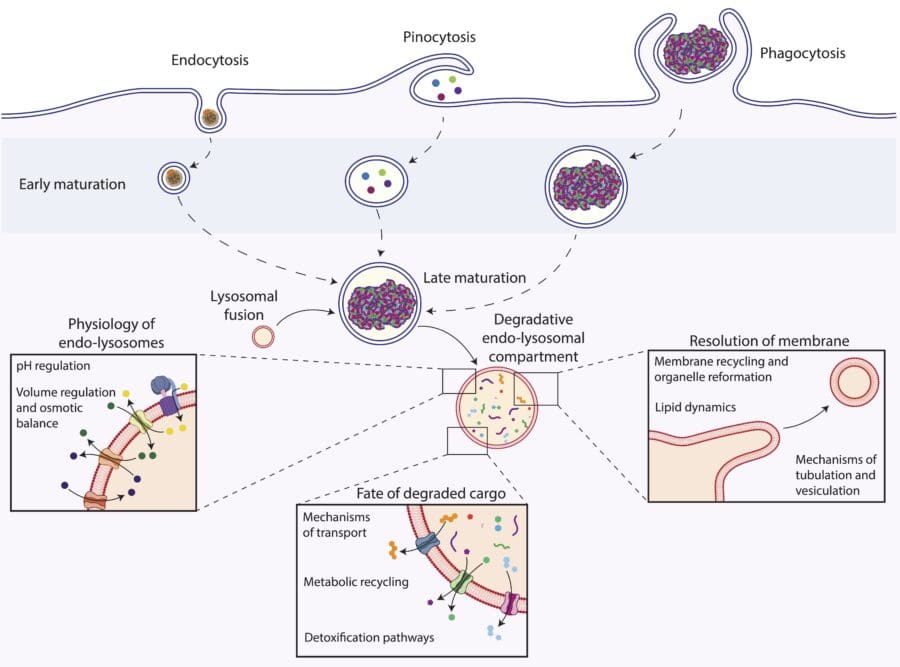
We study this using high-throughput genetic technologies, which allow us to interrogate all the genes in the genome in an unbiased manner. We combine this powerful approach with advanced light microscopy, biochemistry and physiology methods to understand how individual gene products (proteins) of interest work in phagocytes during the clearance of materials. This interdisciplinary, multiscale approach allows us to identify and characterise diverse phenomena, including membrane dynamics, solute transport and phagosomal pH regulation, which are critical for phagocyte function and whose alterations can lead to disease.