Ramanujan Hegde
Membrane protein biosynthesis and quality control

Our group addresses two related questions. First, how do newly-made proteins get to the right part of the cell and assemble into functional products? Second, how do cells recognise errors during protein maturation and target the defective products for degradation? Both processes are essential to all living cells, and even subtle problems in protein maturation or quality control can lead to various diseases.
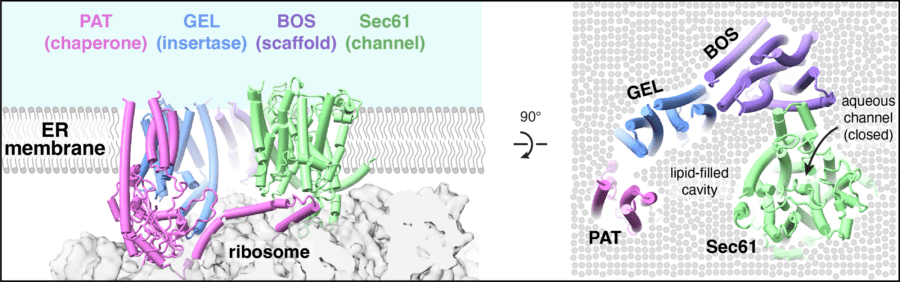
Our work on protein maturation focuses on membrane proteins, which are essential for sensing the environment, communication with other cells, and the transport of nutrients and metabolites. Membrane protein biogenesis involves insertion into the lipid bilayer, folding and assembly with other subunits. To understand how this is accomplished, we take a biochemical approach to identify and mechanistically dissect the factors involved in membrane protein targeting, insertion, folding and assembly. We have developed a new conceptual framework for membrane protein biogenesis based on our findings. Ongoing efforts are aimed at understanding how multi-subunit complexes are assembled.
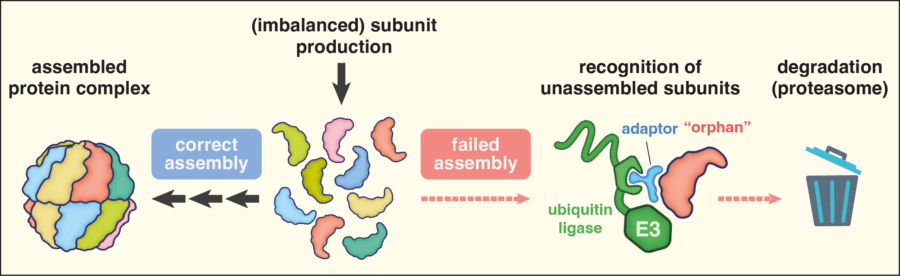
Our work on quality control focusses on proteins that fail to localise to an organelle or fail to assemble into a multi-subunit complex. We have discovered several factors that recognise and dispose of mis-localised or unassembled proteins, which we term orphans. These quality control factors play central roles in maintaining protein homeostasis in the cell, and when mutated, contribute to diseases of protein misfolding such as neurodegeneration. Conversely, they may be particularly crucial for facilitating the rapid growth of mutation-ridden cancer cells in need of robust quality control systems.